Types of Regulated Medical Waste (RMW)
Definitions of "Medical Waste"
Although there is no universally accepted definition for medical waste, the definitions offered by most regulatory agencies are similar. Most federal and state agencies differentiate between common medical waste and those wastes with the potential for causing infection and for which special precautions are prudent. Depending on the state, these wastes are referred to as:
- regulated medical waste (e.g., NY, RI)
- infectious waste (e.g., CO, NE, NV)
- biomedical waste (e.g., CT, FL, GA, ME, WA).
Some state regulations use a general definition, while others list specific wastes and categories of waste that are considered infectious. Some states have adopted the definition found in federal standards (e.g., Nevada adopted the DOT definition).
The following six medical wastes are commonly regulated by states:
-
Pathological waste. Tissues, organs, body parts, and body fluids removed during surgery and autopsy.
-
Human blood and blood products. Waste blood, serum, plasma and blood products.
-
Cultures and stocks of infectious agents (microbiological waste). Specimens from medical and pathology laboratories. Includes culture dishes and devices used to transfer, inoculate, and mix. Also includes discarded live and attenuated vaccines.
-
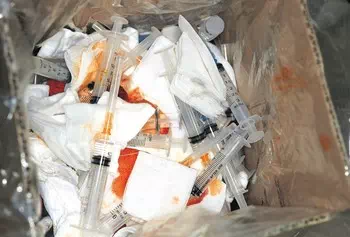
Contaminated sharps. Contaminated hypodermic needles, syringes, scalpel blades, Pasteur pipettes, and broken glass.
-
Isolation waste. Generated by hospitalized patients isolated to protect others from communicable disease.
-
Contaminated animal carcasses, body parts and bedding. From animals intentionally exposed to pathogens in research, biologicals production, or in vivo pharmaceuticals testing.
More detailed discussions of these waste types are provided in the sections below.
Pathology and Anatomy Wastes
Definition: all human anatomical wastes and all wastes that are human tissues, organs, or body parts removed by trauma, during surgery, autopsy, studies, or another hospital procedure, which is intended for disposal.
It is important to understand the distinction between anatomical and pathological waste. While both are wastes derived from the human body, pathological wastes are unique in that these are typically samples of tissues that are examined in a laboratory setting to understand the nature of the disease or affliction from which a patient suffers. For the most part, pathological waste refers to very small tissues sections and body material derived from biopsies or surgical procedures that are then examined in the lab. Anatomical wastes are typically distinguished as recognizable human organs, tissue and body parts, and may require special treatment under some state regulations.
Some states do not consider hair, teeth and nails to be pathological/anatomical waste.
Bulk
human blood, blood products, bulk body fluids or other potentially infectious
material
(OPIM- as defined by OSHA)
Definition: bulk waste human blood, human blood components or products derived from blood including serum, plasma and other blood components, or bulk human body fluids as defined by OSHA to include the following human body fluids: semen, vaginal secretions, cerebrospinal fluid, synovial fluid, pleural fluid, pericardial fluid, peritoneal fluid, amniotic fluid, saliva in dental procedures, any body fluid that is visually contaminated with blood, and all body fluids in situations where it is difficult or impossible to differentiate between body fluids.
This category includes sample of these fluids taken in hematology labs, as well as drainage from surgery, and urine or feces when visibly contaminated by blood.
Microbiological Waste
Definition: cultures and stocks of infectious agents, and associated microorganisms and biologicals. Discarded cultures, culture dishes and devices used to transfer, inoculate and mix cultures, stocks, specimens, live and attenuated vaccines and associated items if they are likely to contain organisms likely to be pathogenic to healthy humans. Discarded etiologic agents and wastes from the production of biologicals and antibiotics likely to have been contaminated by organisms likely to be pathogenic to healthy humans. Waste that originates from clinical or research laboratory procedures involving communicable infectious agents.
Note: Microbiological waste that is also considered a 'sharp' as defined below, should be managed first and foremost as a 'sharp'. It is also important to note what materials your laboratories are working with, as there are special guidelines from CDC on how to handle infectious microorganisms at biosafety level (BSL) 3 and BSL 4. (see Table 1. Summary of BSLs).
Sharps
Definition: Items that can induce subdermal inoculation of infectious agents or that can easily penetrate the skin, puncture waste bags and cardboard boxes, sharps that have been used or are intended to be used in human or animal patient care or in medical, research, or industrial laboratories, including hypodermic needles, syringes, Pasteur pipettes, capillary tubes, broken glass from the laboratory including slides and slide covers, razor blades, and scalpel blades.
Sharps require special handling and packaging under both OSHA and DOT. Be sure to refer to your state’s guidelines when identifying what items are classified as sharps. There is confusion that often needleless injection devices, heel lancers and retractable or needles destruction technologies are considered sharps as well.
Isolation Wastes (Wastes from Highly Communicable Diseases)
Definition: biological waste and discarded materials contaminated with blood, excretion, exudates or secretion from humans or animals who are isolated to protect others from highly communicable diseases (Lassa fever virus, Marburg virus, monkey pox virus, Ebola virus and others (see Table 1. Summary of BSLs).
Animal Waste
Definition: animal carcasses, body parts, bedding and related wastes that may have been exposed to infectious agents during research, production of biologicals, or testing of pharmaceuticals.